View TRITEC® or TRITEC® Silver
Description
These instructions are for TRITEC® and TRITEC® Silver.
TRITEC® and TRITEC® Silver contact layer dressings utilize Active Fluid Management® (AFM®) technology. The three part design combines two layers of high-performance fabrics through a micro-knit process, creating a powerful moisture transfer mechanism that pulls away excess exudate. This patented design keeps the wound bed moist, the periwound skin healthy, and harmful exudate away from healing wounds facilitating healing in hard to heal, highly draining chronic wounds.
TRITEC® Silver dressings provide an antimicrobial barrier to microbial colonization in the dressing and reduce microbial penetration through the dressing. The release of silver into the dressing is activated by the body’s own sodium and begins inactivating pathogens within one hour of dressing application. Antimicrobial activity lasts up to 7 days*.
Indications for Use
TRITEC® and TRITEC® Silver contact layer dressings are indicated for the management of acute and chronic wounds: leg ulcers, pressure ulcers, diabetic foot ulcers, partial thickness burns, skin grafts, incisions, donor sites, lacerations, and abrasions.
Contraindications
TRITEC®: None known
TRITEC® Silver: Should not be used on patients with a known sensitivity to silver.
Precautions & Warnings
- TRITEC® and TRITEC® Silver dressings are not approved for use directly on full thickness burns.
- The Active Fluid Management performance of TRITEC and TRITEC® Silver dressings may be impaired by use with petrolatum-based ointments.
- The use of TRITEC® Silver dressings during radiation treatment or examinations such as X-ray’s, ultrasonic treatment, diathermy, microwaves, MRI or HBO has not been
- Use of TRITEC® Silver dressings during pregnancy, lactation and for pediatric use has not been demonstrated.
- TRITEC® and TRITEC® Silver dressings are sterile as provided. Do Not use if the primary package is visually damaged or broken.
- Do Not resterilize.
- Do Not Reuse. TRITEC® and TRITEC® Silver dressings are single use products.
- Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner (U.S.A. only).
Directions for Use
General Instructions:
TRITEC® and TRITEC® Silver dressings can be used throughout the healing process.
- Prepare the wound bed according to clinical protocol such as debridement and/or cleaning and rinsing with sterile saline or water.
- Remove the dressing from the package and apply to a highly exudating wound with the printed side of the dressing facing away from the wound.
(2) Secure with an appropriate secondary dressing such as roll gauze, adhesive border gauze, composite island dressing, transparent film, or tape if needed.
NOTES
- Optimal performance requires direct contact with the wound.
- Can be used under compression.
- For dry wounds you can place the dressing print side towards the wound with a source of fluid behind to add moisture to the wound.
Applying TRITEC Dressings
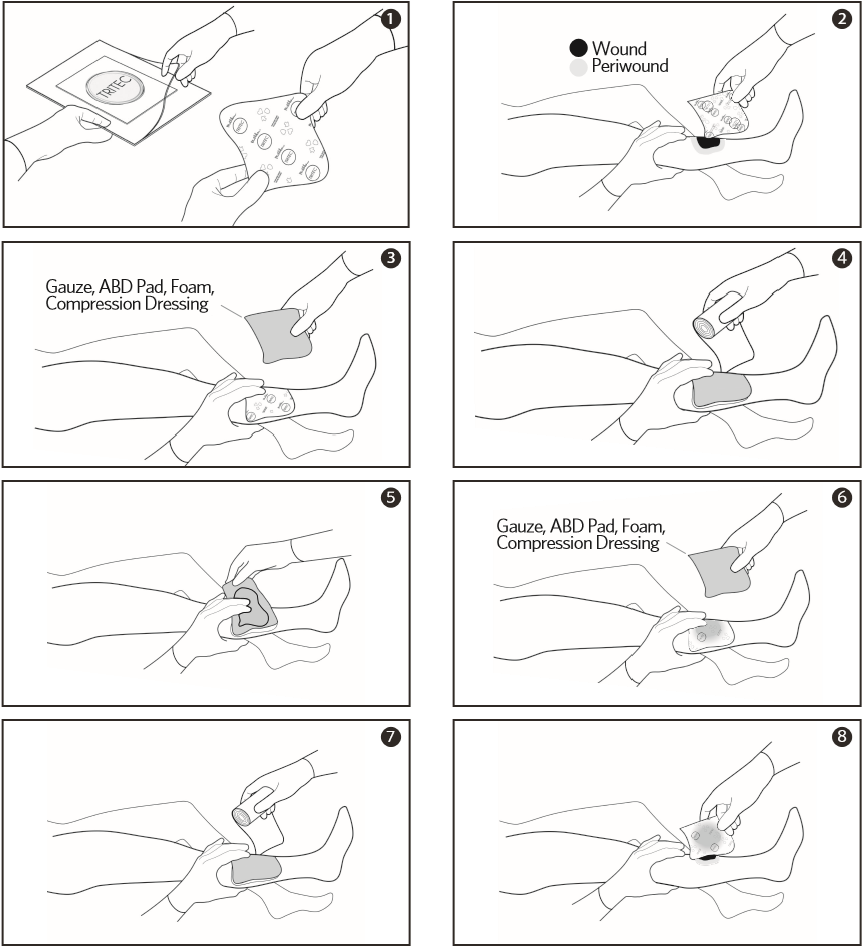
- Remove TRITEC® dressing from package. If required, dressing can be cut to size.
- Apply TRITEC® with printed side of dressing away from the wound.
- Add absorbent secondary dressing on top of the TRITEC® dressing (e.g. gauze, ABD pad, foam, compression dressing).
- Secure both dressings using standard methods.
- If secondary dressing becomes saturated, discard saturated secondary dressing without disturbing TRITEC® dressing.
- Add a new absorbent secondary dressing on top of the TRITEC® dressing (e.g. gauze, ABD pad, foam, compression dressing).
- Re-Secure both dressings using standard methods.
- Replace TRITEC® dressing per doctor’s orders. Gently lift dressings from the wound. If needed, moisten with sterile saline or water to ease removal.
Removal
TRITEC® and TRITEC® Silver dressings should be changed when clinically indicated or as prescribed by a healthcare professional. Carefully remove the fixative and the dressing from the wound. Normally the dressing does not adhere to the wound. If adherence is observed, the dressing can be moistened with sterile saline or water to ease removal.
Storage
For best results, store in original packaging at room temperature less than 131˚F until use. Avoid excessive heat and humidity. Refer to the label on each box for expiration date.
Made in U.S. by OVIK Health, LLC #

Spartanburg, South Carolina
www.ovikhealth.com · 800.432.6686
*Data on file
© 2023 OVIK Health, LLC All rights reserved.
Document # OVIKPPr0 2024-01
View TRITEC® or TRITEC® Silver

